AFM Systems
AFM Accessories
Learning
Contact Us
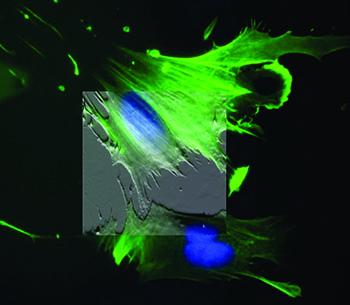
Atomic force microscopy is an essential tool for cell biology research. It can provide 3D topographical data of living, unfixed cells. However, AFM’s greatest strength in cell biology is its ability to provide accurate and quantitative mechanical measurements in near-physiological conditions (i.e. in culture medium and at 37°C). The elastic and viscoelastic response of a cell or substrate can be routinely measured using force maps and AFM-based microrheology techniques, respectively. The measured cell moduli can be that of unaltered cells, cells in different states of development, differentiation, or disease, or cells responding to a stimulus such as a drug or mechanical stress. Measuring the moduli of substrates and the cell microenvironment is also important due to the role the extracellular matrix (ECM) plays in such processes as cell differentiation, fate, signalling, gene transcription, cancer, cardiovascular disease and apoptosis.
When integrated with an inverted optical microscope (i.e. fluorescence, confocal, TIRF, etc.), data from both imaging modalities can be combined to correlate fluorescently labeled structures with AFM topography. The optics can be used to direct the AFM tip to probe a particular region of the cell, which can be crucial for hard-to-image cell types. Finally, AFM can also be used to provide a mechanical stimulus to cells and the associated response (e.g. ion handling, membrane potential changes, etc.) can be recorded optically in order to understand mechanotransduction in living cells and tissues.
"Peptide density targets and impedes triple negative breast cancer metastasis," D. Liu, P. Guo, C. McCarthy, B. Wang, Y. Tao, and D. Auguste, Nat. Commun. 9, 2612 (2018). https://doi.org/10.1038/s41467-018-05035-5
"Direct micropatterning of extracellular matrix proteins on functionalized polyacrylamide hydrogels shows geometric regulation of cell–cell junctions," B. Sarker, C. Walter, and A. Pathak, ACS Biomater. Sci. Eng. 4, 2340 (2018). https://doi.org/10.1021/acsbiomaterials.8b00331
"Measuring nanoscale viscoelastic parameters of cells directly from AFM force-displacement curves," Y. M. Efremov, W.-H. Wang, S. D. Hardy, R. L. Geahlen, and A. Raman, Sci. Rep. 7, 1541 (2017). https://doi.org/10.1038/s41598-017-01784-3
"3D cell bioprinting of self-assembling peptide-based hydrogels," B. Raphael, T. Khalil, V. L. Workman, A. Smith, C. P. Brown, C. Streuli, A. Saiani, and M. Domingos, Mater. Lett. 190, 103 (2017). https://doi.org/10.1016/j.matlet.2016.12.127
"T cell activation requires force generation," K. H. Hu and M. J. Butte, J. Cell Biol. 213, 535 (2016). https://doi.org/10.1083/jcb.201511053
"Mechanical heterogeneities in the subendothelial matrix develop with age and decrease with exercise," J. C. Kohn, A. Chen, S. Cheng, D. R. Kowal, M. R. King, and C. A. Reinhart-King, J. Biomech. 49, 1447 (2016). https://doi.org/10.1016/j.jbiomech.2016.03.016
"Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration," I. Acerbi, L. Cassereau, I. Dean, Q. Shi, A. Au, C. Park, Y. Y. Chen, J. Liphardt, E. S. Hwang, and V. M. Weaver, Integr. Biol. 7, 1120 (2015). https://doi.org/10.1039/c5ib00040h
"Nanomechanics of cells and biomaterials studied by atomic force microscopy," J. I. Kilpatrick, I. Revenko, and B. J. Rodriguez, Adv. Healthcare Mater. 4, 2456 (2015). https://doi.org/10.1002/adhm.201500229
"Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression," J. K. Mouw, Y. Yui, L. Damiano, R. O. Bainer, J. N. Lakins, I. Acerbi, G. Ou, A. C. Wijekoon, K. R. Levental, P. M. Gilbert, E. S. Hwang, Y.-Y. Chen, and V. M. Weaver, Nat. Med. 20, 360 (2014). https://doi.org/10.1038/nm.3497
"Atomic force microscopy-based microrheology reveals significant differences in the viscoelastic response between malign and benign cell lines," J. Rother, H. Nöding, I. Mey, and A. Janshoff, Open Biol. 4, 140046 (2014). https://doi.org/10.1098/rsob.140046
"A physical sciences network characterization of non-tumorigenic and metastatic cells," The Physical Sciences – Oncology Centers Network (D. B. Agus et al.), Sci. Rep. 3, 1449 (2013). https://doi.org/10.1038/srep01449
"Comparison of the viscoelastic properties of cells from different kidney cancer phenotypes measured with atomic force microscopy," L. M. Rebelo, J. S. de Sousa, J. Mendes Filho, and M. Radmacher, Nanotechnology 24, 055102 (2013). https://doi.org/10.1088/0957-4484/24/5/055102
"Elasticity maps of living neurons measured by combined fluorescence and atomic force microscopy," E. Spedden, J. D. White, E. N. Naumova, D. L. Kaplan, and C. Staii, Biophys. J. 103, 868 (2012). https://doi.org/10.1016/j.bpj.2012.08.005
"Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells," W. Xu, R. Mezencev, B. Kim, L. Wang, J. McDonald, and T. Sulchek, PLoS One 7, e46609 (2012). https://doi.org/10.1371/journal.pone.0046609
"In situ force mapping of mammary gland transformation," J. I. Lopez, I. Kang, W.-K. You, D. M. McDonald, and V. M. Weaver, Integr. Biol. 3, 910 (2011). https://doi.org/10.1039/c1ib00043h
"Mapping nanomechanical properties of live cells using multi-harmonic atomic force microscopy," A. Raman, S. Trigueros, A. Cartagena, A. P. Z. Stevenson, M. Susilo, E. Nauman, and S. A. Contera, Nat. Nanotechnol. 6, 809 (2011). https://doi.org/10.1038/nnano.2011.186
"Detection of single-molecule H2O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes," H. Jin, D. A. Heller, M. Kalbacova, J.-H. Kim, J. Zhang, A. A. Boghossian, N. Maheshri, and M. S. Strano, Nat. Nanotechnol. 5, 302 (2010). https://doi.org/10.1038/nnano.2010.24
"Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells," E. K. Yim, E. M. Darling, K. Kulangara, F. Guilak, and K. W. Leong, Biomaterials 31, 1299 (2010). https://doi.org10.1016/j.biomaterials.2009.10.037
"Impact of actin rearrangement and degranulation on the membrane structure of primary mast cells: A combined atomic force and laser scanning confocal microscopy investigation," Z. Deng, T. Zink, H. Chen, D. Walters, F. Liu, and G. Liu, Biophys. J. 96, 1629 (2009). https://doi.org/10.1016/j.bpj.2008.11.015
"Control of myocyte remodeling in vitro with engineered substrates," N. A. Geisse, S. P. Sheehy, and K. K. Parker, In Vitro Cell. Dev. Biol.–Animal 45, 343 (2009). https://doi.org/10.1007/s11626-009-9182-9
"Intermediate filament-like proteins in bacteria and a cytoskeletal function in Streptomyces," S. Bagchi, H. Tomenius, L. M. Belova, and N. Ausmees, Mol. Microbiol. 70, 1037 (2008). https://doi.org/10.1111/j.1365-2958.2008.06473.x
"A cell nanoinjector based on carbon nanotubes," X. Chen, A. Kis, A. Zettl, and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A. 104, 8218 (2007). https://doi.org/10.1073/pnas.0700567104
"Matrix elasticity directs stem cell lineage specification," A. J. Engler, S. Sen, H. L. Sweeney, and D. E. Discher, Cell 126, 677 (2006). https://doi.org/10.1016/j.cell.2006.06.044
"Indentation and adhesive probing of a cell membrane with AFM: Theoretical model and experiments," S. Sen, S. Subramanian, and D. E. Discher, Biophys. J. 89, 3203 (2005). https://doi.org/10.1529/biophysj.105.063826
"Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments," A. J. Engler, M. A. Griffin, S. Sen, C. G. Bönnemann, H. L. Sweeney, and D. E. Discher, J. Cell Biol. 166, 877 (2004). https://doi.org/10.1083/jcb.200405004