AFM Systems
AFM Accessories
Learning
Contact Us
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Although nanoparticles (NPs) have been used for drug delivery since the 1990s, recent advances in nanotechnology have enabled researchers to develop novel structures with improved properties. Nanoparticles now play an increasingly important role in cancer treatment, gene therapy, and the treatment of neurodegenerative diseases. An effective drug delivery vehicle should be biocompatible and nontoxic, provide improved solubility and bioavailability of the drug, protect and transport the drug to the target site (cell or tissue), and deliver the controlled release of the drug at its destination.1-2
Several analytical techniques are commonly used to characterize NPs including dynamic light scattering (DLS), transmission electron microscopy (TEM), cryo-electron microscopy (cryo-EM), and light microscopy; however, each has its limitations. DLS can provide a size distribution, but because it assumes that the NPs have a spherical structure, it cannot distinguish between different morphologies. TEM and cryo-EM are electron microscopies that operate in a non- native environment and typically require a conductive coating to be applied to the sample. This limits the type of samples and environments that can be explored. Finally, light microscopy cannot provide sufficient resolution if the particle size is below the diffraction limit of light. Atomic force Microscopy (AFM) circumvents many of these limitations and provides additional measurement capabilities to more fully characterize drug delivery NPs. AFM can accurately determine the morphology and size distribution of the entire population of NPs in an aqueous environment. It can also measure the nanomechanical properties of the NPs which can be used to further optimize their composition and formulation. And with the improved temporal resolution of high-speed AFM, dissolution rates and degradation mechanisms of fast-dissolving nanoparticles can be determined.
Nanoparticles for drug delivery are categorized by their composition: lipid nanoparticles (LNPs) and polymer nanoparticles (PNPs). These two categories can be further sub-divided based on their lipid arrangement and core content. Each type of structure has its advantages and its appropriate applications. In this report, we will focus on liposomes and amorphous nanoparticles.
Liposomes can be tailored for optimal interaction with their target making them highly versatile drug delivery vehicles. Since they contain both hydrophobic and hydrophilic regions, they can be designed to carry hydrophilic and lipophilic drugs in either their aqueous or lipid leaflet regions, respectively. Liposomes have superior therapeutic effectiveness due to their excellent biocompatibility, minimal toxicity, stability, and improved absorption. To optimize performance, it is important to characterize their size and morphology.1,3
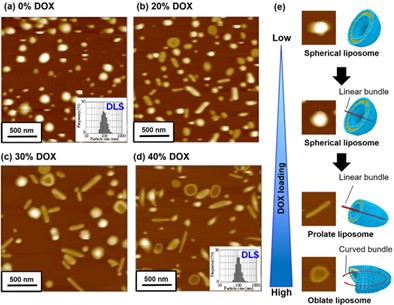
Figure 1: Topography and observed shape of Doxyl liposomes as a function of incorporated drug content: (a) 0%, (b) 20%, (c) 30%, and (d) 40%. 2 µm scan. DLS particle size distribution histograms for (a) and (d) are shown in the insets. (e) Individual liposome images and cartoons for each shape.
Doxorubicin (DOX) is one of the strongest chemotherapy drugs. It works by inhibiting DNA synthesis and triggering the production of free radicals at the tumor site. To determine the liposome size distribution and shape, Doxorubicin (DOX)-encapsulated liposomes in their aqueous phase were imaged with AFM as a function of DOX inclusion (drug loading). The AFM data reveals an increase in shape diversity as the amount of incorporated drug is increased, even though the average particle size determined using DLS (Figure 1a,d inset) remained constant at approximately 100 nm, with a range of 50 – 200 nm. With empty or 10% inclusion, the liposomes were spherical (Figure 1a). However, with higher drug loading, the liposomes became increasingly elongated, forming either prolate or oblate structures as shown in Figure 1b-d. At the highest loading of 30-40% there was also a greater proportion of liposomes with a depressed center. These structural changes are a consequence of the drug arrangement inside the liposomes. DOX forms long fibers that become increasingly elongated at higher concentrations, eventually bending to form curved bundles.4-5
Amorphous nanoparticle formulations are relatively new to drug delivery but are gaining interest due to their ability to improve both the solubility and bioavailability of poorly soluble drugs. Their main disadvantage is that they are thermodynamically unstable and prefer to be in their more stable crystalline state. Crystallization time can vary and there are options to prevent or slow this process, but further research is required to fully understand the mechanisms involved.6-8
AFM was used to study the nanomechanical properties of amorphous Probucol (PBC) nanoparticles covered by water-soluble Hypromellose (HPMC) polymer. Force-separation (indentation) measurements were used to distinguish between amorphous, semi-crystalline, and completely crystalline states of the NPs as a function of time. By pushing the AFM probe into the NP surface and monitoring the cantilever deflection, the stiffness of the NP can be determined. In Figure 2, we see differences in the force-separation curves between softer, smaller, “young” amorphous NPs (a), stiffer, larger, “older” semi-crystalline NPs (b, c), and very stiff, largest, “oldest” crystalline NPs (d).7 A force-separation measurement was also made on the bare substrate for comparison (Figure 2S).
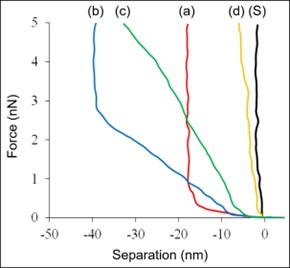
Figure 2: Force vs. separation curves for NP samples at: (a) 0-1 day, (b) 2 days, (c) 4 days, and (d) 7 days. (S) was acquired on the bare substrate.
In Figure 2, the slope of curves (a) and (b) starts low, indicating a compliant sample, but exhibits an abrupt increase at -17 nm and -40 nm indentation depth, respectively.
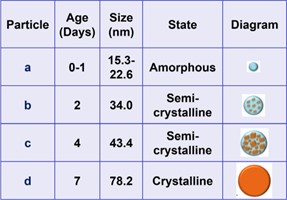
Table 1: Size, state, and proposed structure of amorphous polymer-coated PBC NP samples as a function of time (0-7 days).
This behavior may be attributed to substrate effects that are prominent with individual NPs or small clusters but diminish with age, increased particle size, and stiffness. Table 1 compares the NP age, particle size, and state, and includes proposed structures for each case.
In addition to improving the solubility of poorly soluble drugs, polymer nanoparticles are environmentally responsive nanocarriers that can release their incorporated drug in response to changes in buffer composition, pH, or temperature. In particular, pH is an ideal stimulus since vesicles for cell internalization and tumors tend to have lower pH.9 However, the PNPs tend to dissolve quickly, making it challenging to measure their dissolution rates with conventional methods.10 High-speed AFM is a powerful tool that allows us to observe and capture changes in the PNP morphology, as well as the eventual dissolution and release of the encapsulated drug. Figure 3 consists of a series of images acquired in liquid at 1 frame per second (FPS). Mefenamic acid (MFA, model drug)- encapsulated Eudragit® E (EUD-E) PNPs were designed to dissolve in acidic solutions at pH5. Within three seconds, the PNPs began to decrease in size and completely disappeared after five seconds. This experiment emphasizes the importance of evaluating the dissolution process in relevant environments (temperature, pH, buffer) ‘in vivo’ to assess the quality of the nanoparticles and the accuracy of the dissolution to ensure the drug reaches its target site before release.
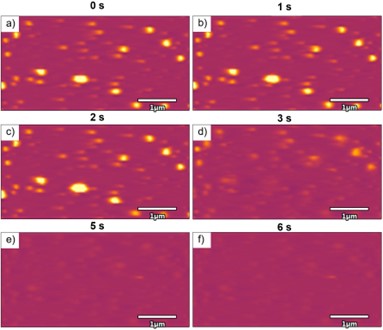
Figure 3: Dissolution of Mefenamic acid-encapsulated Eudragit E polymer nanoparticles under acidic solutions (pH 5) after exposure for a) 0 s, b) 1 s, c) 2 s, d) 3 s, e) 5 s, and f) 6 Acquired with the Cypher VRS1250. 4 x 2 µm scans.
AFM is a powerful tool for characterizing the morphology and nanomechanical properties of lipid and polymer nanoparticles in a native, liquid environment. Furthermore, high-speed AFM improves our understanding of fast-acting NPs by revealing real- time dissolution dynamics. Results from these types of studies can be used in process optimization to improve nanoformulation recipes and NP drug delivery efficacy.
If you have any questions about this note please contact AFM.info@oxinst.com to speak with one of our experts.
Oxford Instruments Asylum Research. The materials presented here are summary in nature, subject to change and intended for general information only. Performances are configuration dependent. © Oxford Instruments plc, 2024. All rights reserved. Do not reproduce without permission.
All rights reserved. LITR511990-01
© Oxford Instruments plc, 2024.
Author: Asylum Research
Category: Application Note
