AFM Systems
AFM Accessories
Learning
Contact Us
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Crystallized anti-inflammatory drugs inhibited fibrosis on the surface of medical implants over long periods of time. AFM time-resolved images gave insight into the mechanisms of drug release on the molecular level.
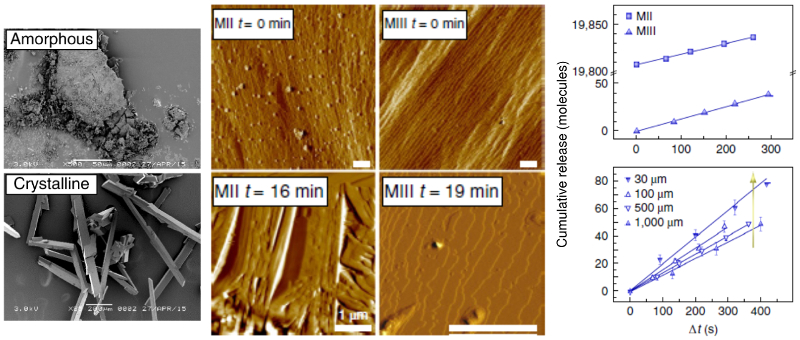 Implantable devices are a critical part of many medical treatments. However, they are often attacked by the immune system after insertion. The resulting buildup of scar tissue, called fibrosis, can reduce the device’s functionality and even cause failure.
Implantable devices are a critical part of many medical treatments. However, they are often attacked by the immune system after insertion. The resulting buildup of scar tissue, called fibrosis, can reduce the device’s functionality and even cause failure.
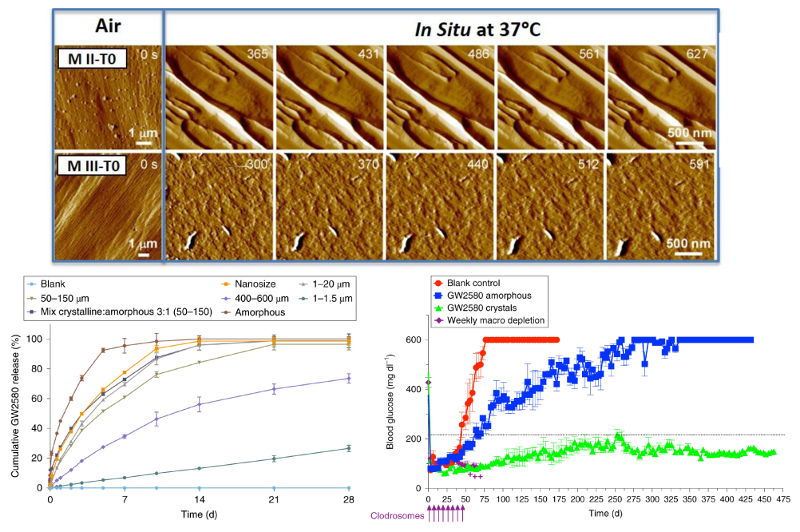
A U. S. team led by researchers at the Massachusetts Institute of Technology has shown how to limit fibrosis by incorporating a crystallized immunosuppressant drug in the devices. The crystallized drugs release slowly over time and locally dampen the immune response that causes fibrosis. As part of the study, time-resolved AFM imaging was used to measure the release rate of drug molecules and the dependence on crystal size and type.
In tests on rodents and non-human primates, the technique significantly enhanced performance. For instance, implants to control diabetes stayed fibrosis-free for over a year. Other applications that could benefit from these results include biomedical devices like pacemakers, stents, and sensors.
Cypher ES with the Liquid Perfusion Cell
Topography images of drug crystal morphology were acquired on the Cypher ES AFM in both tapping mode and contact mode. Imaging was performed under both ex situ (air at 25 °C) and in situ (phosphate buffered saline, PBS, solution at 37 °C) conditions. For the in situ drug release studies, sequences of molecular-level images were acquired ~1 minute apart. Constant temperature was maintained throughout the experiments using the superb temperature control capabilities of the Cypher ES. The ES’s sealed Liquid Perfusion Cell enabled controlled operation in liquids and was specifically designed to accommodate harsh solvents.
Citation: S. Farah, J. Doloff, P. Müller et al., Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat. Mater. 18, 892 (2019). https://doi.org/10.1038/s41563-019-0377-5
Note: The data shown here are reused under fair use from the original article, which can be accessed through the article link above.
Author: Asylum Research
Category: Technical Article
