AFM Systems
AFM Accessories
Learning
Contact Us
 Part of the Oxford Instruments Group
Part of the Oxford Instruments Group
Complementary information from in-situ AFM, in-situ x ray scattering, and ex-situ scanning electron microscopy (SEM) revealed how the morphology of zinc evolves during electrodeposition in an ionic liquid electrolyte.
Zinc (Zn) is an attractive option for rechargeable battery electrodes and other energy storage applications, but it tends to form filaments, nodules, and dendrites that short circuit the cell. Ionic liquid electrolytes have been found to reduce this behavior. However, deeper understanding is needed of the mechanisms that control deposition and morphology.
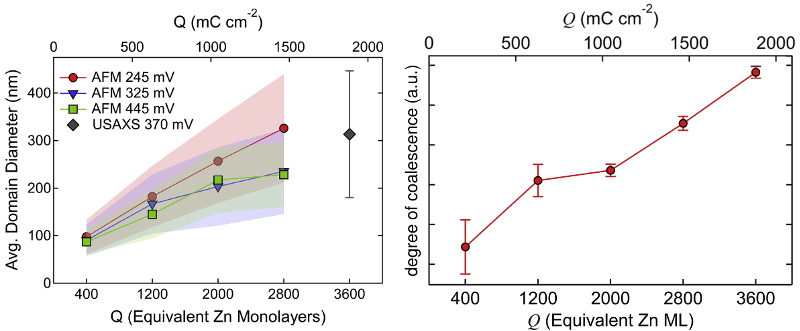
To that end, researchers at the University of California–Berkeley and Lawrence Livermore National Laboratory studied electrodeposition of Zn in an imidazolium-based electrolyte [1-butyl-3-methyl-imidazolium (BMIm) cation and trifluoromethanesulfonate (TfO) anion]. They used in-situ AFM, in-situ ultra-small angle X-ray scattering (USAXS), and ex-situ SEM to characterize film morphology versus thickness in terms of charge Q and equivalent Zn monolayers (ML).
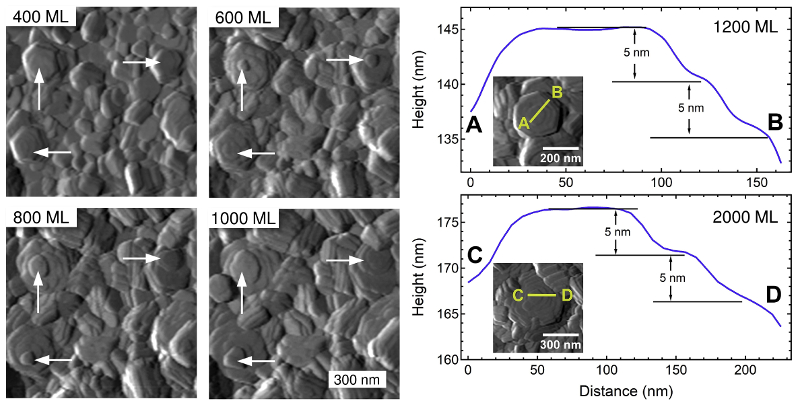
Two main structures were apparent: hexagonal plates of single crystals and larger domains with co-aligned crystals. AFM and SEM imaging allowed measurement of features such as plate thickness and domain size over time. The information also guided interpretation of USAXS data, which is more statistically averaged but requires fitting to a direct-space model. This complementary approach yielded new insight into morphological progress and mechanisms such as platelet growth and coalescence.
The results provide a more complete picture of Zn electrodeposition that could improve cyclability of Zn-electrode batteries and thus make them commercially practical.
MFP-3D AFM with an Electrochemistry Cell
The morphology of electrodeposited zinc was monitored in situ on an MFP-3D AFM with an Electrochemistry (EC) Cell. The EC Cell provides a versatile environment for a wide range of electrochemistry studies. Topography images were acquired in contact mode periodically during film growth. A port on the cell allowed argon gas to be percolated during the experiments to improve control of electrolyte moisture.
Citation: J. Keist, J. Hammons, P. Wright et al., Coupling in situ atomic force microscopy (AFM) and ultra-small-angle X-ray scattering (USAXS) to study the evolution of zinc morphology during electrodeposition within an imidazolium based ionic liquid electrolyte. Electrochim. Acta 342, 136073 (2020). https://doi.org/10.1016/j.electacta.2020.136073
Note: The data shown here are reused under fair use from the original article, which can be accessed through the article link above.
